The size of an organ or tissue is determined both by the number of cells it has and the size of each of those cells. The increase in size during development and thereafter is thought to occur in one of two ways: ‘hyperplasia’ or ‘hypertrophy’. Hyperplasia is when a tissue grows by increasing cell number, for example, the enlargement of breast tissues during pregnancy. Hypertrophy is when a tissue grows by increasing cell size, for example, the skeletal muscles in response to resistance training. There are also some instances in which the increase in organ size involves both processes. For example, after surgical removal of a part of the liver, some cells of the liver increase in number and while others increase in size to facilitate liver regeneration. Therefore, it becomes important to study how a cell chooses to engage in either of these processes as ‘dysregulation’ can lead to improper organ formation or repair; further triggering the onset of disease like cancer.
The fruit fly, Drosophila melanogaster, is an excellent system to study how cells switch between hypertrophy and hyperplasia. During the course of development, Drosophila transforms from a larva into an adult fly. As the adult form is very different from the larval form, the fly almost literally makes itself again from small patches of ‘progenitor’ cells set aside in the larval body. Arjun Guha’s lab at Regulation of Cell Fate (RCF) theme, inStem focuses on studying a subset of the progenitor cells that give rise to the respiratory system of the adult fly. These airway progenitors, hereafter called ‘tracheoblasts’, form a part of the growing larval respiratory/tracheal system and also contribute toward generation of the adult respiratory structures. Tracheoblasts are unique in their ability to form functional structures in the larva and still maintain the potential to contribute to the generation of adult organs. They pause the cell division in the larval stages during which period the cells grow in size (hypertrophy), and then restart cell division and divide rapidly (hyperplasia).
In order to understand how tracheoblasts coordinate the increase in size and number, it is important to familiarize oneself with the concept of the cell cycle. It consists of a growth phase, G1, a synthesis phase, or the S phase, where the cell replicates its DNA, followed by another growth phase, G2, and finally the cell divides by undergoing mitosis (M phase). For cells to be able to grow in size, they need to be actively engaged in the cell cycle. Cells that exit the cycle are called ‘quiescent’ and these cells typically do not divide or grow in size.
Previous studies from the lab have revealed that tracheoblasts pause for between 48 and to 56 hours in the G2 phase of the cell cycle. Other studies have reported that Drosophila progenitors pause due to the lack a protein essential for mitosis called Cdc25/String. We, however, observed that tracheoblasts continue to produce ‘String’ even when they are paused. We showed that these cells pause because they also make another protein, known as Checkpoint Kinase 1/Grapes (Chk1/Grp). As expected, when Chk1 was removed, it reactivated the paused cells sooner, but resulted in smaller cells and a smaller respiratory organ.
An important aspect of the tracheoblasts that are paused in G2, is that they make all the necessary drivers to progress to mitosis and also make a ‘brake’, Chk1. Removing any of the positive drivers or Chk1 from these cells lead to smaller cell and organ size. Thus, the optimal hypertrophic growth of tracheoblasts requires the juxtaposition the drivers of G2-M (commitment to cell cycle), and Chk1 (to prevent cell division).
As indicated earlier, the role of Chk1 as a cell cycle ‘brake’ is widely recognized. “Dividing cells subject to DNA damage are known to activate Chk1 to pause the cell cycle in an attempt to repair the damage prior to resuming the cell cycle. Interestingly, tracheoblasts do not show evidence of any DNA damage. Thus, the mechanism for Chk1 activation was unclear,” says Amrutha, the primary researcher.
“Cells act in response to chemical signals in their vicinity. These signals, or ligands, bind to receptors on the cell surface, triggering a cascade that finally result in expression of a gene. The recent study published in eLife, shows that in the case of tracheoblasts, Wnt, a key developmental signal promotes high levels of Chk1 activity. Removing Wnt leads to the loss of Chk1 in cells. In fruit fly there are seven types of Wnt ligands, of which four are present in the tracheoblasts. All four ligands are necessary for maintaining the levels of Chk1 in these cells and removal of any of them leads to a loss of Chk1 and thus, early division,” quotes the research group.
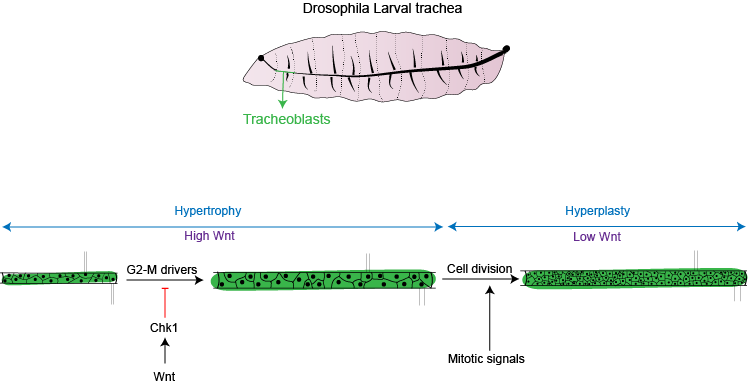
“This work extends our understanding of what cells require for hypertrophic growth (juxtaposition of positive and negative regulators of the cell cycle) and how switch from hypertrophic to hyperplastic growth (removing the negative regulator). We show that signals like Wnt can maintain the cells in a hypertrophic growth phase. Once Wnt is removed, cells enter division and are now able to respond to mitotic signals and grow in numbers,” says Dr. Guha. A fascinating finding of this work, as per the team, is while the cells have high Wnt/Chk1 and are paused in G2, these cells also are unable to respond to signals that promote division. This goes to show how signals in a cellular environment instruct cells to stop and start cell cycle; thereby facilitating growth in cell size and/or number. Interestingly, the role of Wnts in the regulation of Chk1 could also be relevant to cancer cells that are resistant to chemotherapy and radiation since high Chk1 expression can protect against DNA damage.
Reference:
Multiple Wnts act synergistically to induce Chk1/Grapes expression and mediate G2 arrest in Drosophila tracheoblasts
Amrutha Kizhedathu, Rose Sebastian Kunnappallil, Archit V Bagul, Puja Verma, Arjun Guha Sept 2020, eLife
https://elifesciences.org/articles/57056
Publication Date: Sept 22, 2020
Artwork: Amrutha Kizhedathu










