Cells naturally experience fluctuating nutrient conditions. To adapt and grow in such conditions, cells precisely balance the allocation of available nutrients through finely regulated metabolism. This regulation occurs through the functioning of nutrient responsive signaling-systems. The two important signaling complexes in all eukaryotes that regulate nutrient utilization and cell growth are target of rapamycin (TORC1) and AMP-activated protein kinase (AMPK). The TOR kinase gets activated in the presence of nutrients and promotes growth. Conversely, AMPK responds to energy stress, glucose depletion, and activates catabolic processes. TORC1 and AMPK are active under different physiological conditions and regulate opposing cellular processes. However, few studies have shown that inhibition of both these complexes leads to impaired growth. How the inhibition of complexes with opposite function leads to impaired growth? There is a complex regulation involved rather than just the contrary roles of these complexes.
In an effort to address this issue, Zeenat Rashida, Rajalakshmi Srinivasan, and Meghna Cyanam from Dr. Sunil Laxman’s lab at Regulation of Cell Fate theme, DBT-inStem used baker’s yeast i.e. Saccharomyces cerevisiae. Their study discovered a novel role of a protein called Kog1 in yeast (and Raptor in mammals). This protein holds the TOR complex together, and appeared to be critical for activating AMPK in yeast, to regulate carbon allocation towards different metabolic pathways. This is one of the first studies showing how SNF1 and TORC1 can work together to regulate metabolism and growth, in changing nutrient environments.
This discovery began with a chance identification of a mutant of Kog1 that shows growth defects specifically under conditions of glucose and amino acid limitation, where TORC1 kinase activity is very low. For cells to grow in such nutrient-limiting conditions the pathway of gluconeogenesis, TCA cycle and amino acids biosynthesis are important. Using this mutant, the authors found a very specific metabolic rewiring, where carbon allocation towards amino acids biosynthesis was increased, while gluconeogenesis was decreased. A series of subsequent experiments revealed that this was due to defective activation of the yeast AMP kinase.
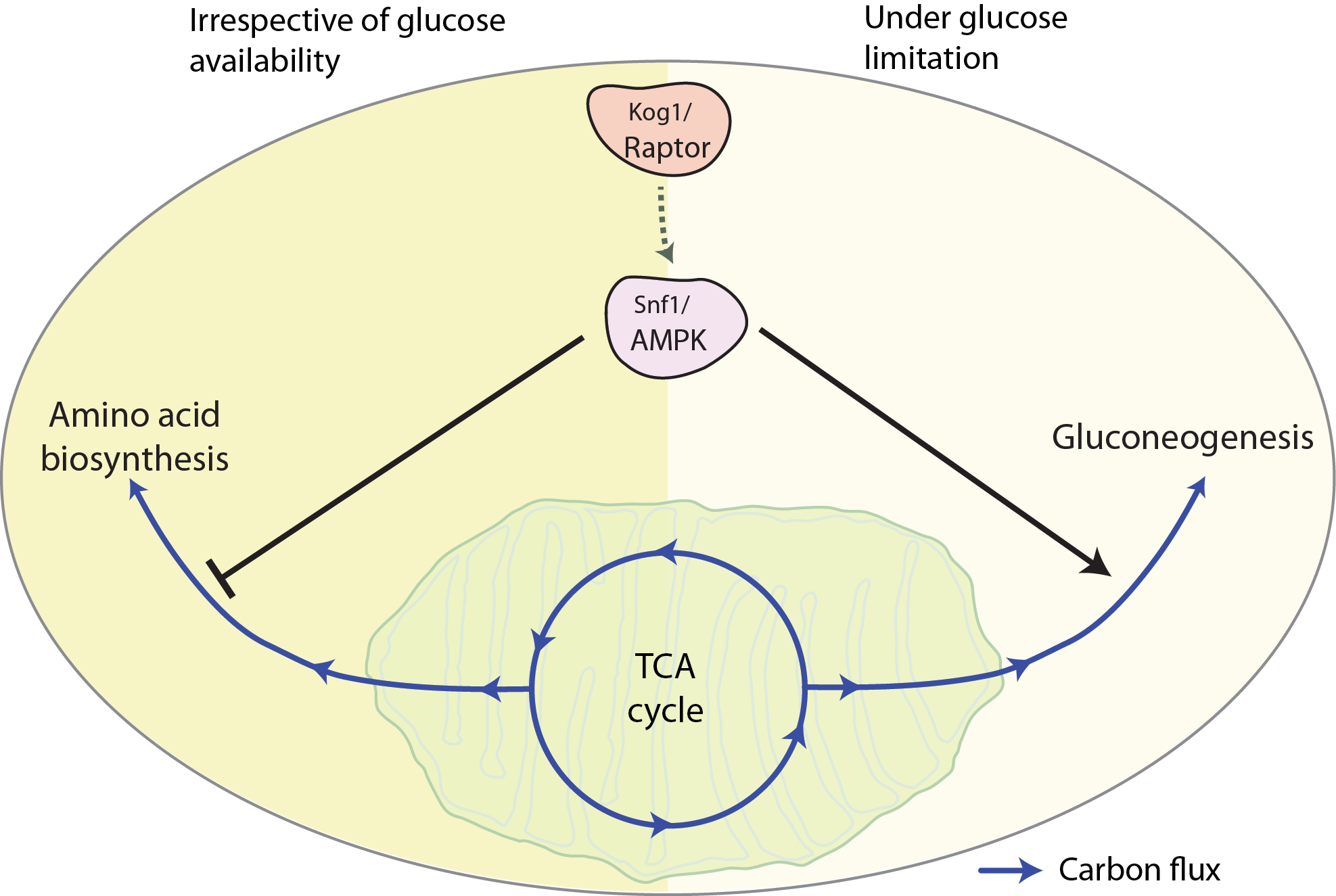
Image: A model showing how Raptor and AMPK together balance carbon flux through two metabolic pathways- amino acid biosynthesis and gluconeogenesis, especially in changing nutrient environments, to enable cells to grow.
AMPK regulates the activation of multiple metabolic pathways that are essential for growth during glucose limitation. In such conditions, it activates the process of gluconeogenesis by regulating the activity of important downstream transcription factors. In the mutant cells, activation of these downstream targets was perturbed, which indicated that the activity of the yeast AMPK depended on a function of Kog1/RAPTOR in regulating AMPK activity through a novel mechanism. A combination of experimental approaches then revealed that this RAPTOR and AMPK dependent control of carbon homeostasis, with a careful allocation of resources towards amino acid biosynthesis, was universal; but most critical for growth when nutrient environments changed. The study also found that AMPK is downstream of RAPTOR, and also was entirely independent of TORC1 kinase activity.
Thus, AMPK and TORC1 work in concert to control cellular metabolism to enable growth. This study now clarifies many long-standing puzzles observed from many different systems, including tumor environments. It is now emerging that both TORC1 activity and AMPK activity are required to sustain tumor growth. Additionally, there are hints of synergistic roles of both TORC1 and AMPK to help cells to sustain growth in what appear to be settings where nutrients are not plentiful. Finally, many studies hint at roles for TORC1 beyond the well-studied kinase dependent functions. This study, therefore, is important because it shows unambiguously that TORC1 and AMPK can function together to help cells grow; but it is not through the conventional kinase dependent roles of TORC1. More generally, it allows us to conceptualize and understand how cells manage their available resources in changing nutrient environments and still manage to grow.
Article credit: Shreyas Niphadkar
Reference:
Kog1/Raptor mediates metabolic rewiring during nutrient limitation by controlling SNF1/AMPK activity
Zeenat Rashida, Rajalakshmi Srinivasan, Meghana Cyanam and Sunil Laxman, Apr 2021, Science Advances
https://advances.sciencemag.org/content/7/16/eabe5544
Publication Date: Apr 14, 2021











