Skin is the largest and the outermost organ of the body and serves as a barrier from the external environment. It has two compartments – the epidermis and dermis. The two compartments are separated by an array of extracellular matrix (ECM) components known as the basement membrane (BM). The ECM is a meshwork of macromolecules such as proteins and carbohydrates that aid in maintaining the structure and integrity of a tissue. The skin cells together with their repertoire of white blood cells (immune cells) protect internal organs against mechanical, chemical, UV, and biological damage.
Immune cells can be broadly categorized into innate and adaptive cells. During wounding, the damaged cells within the tissue release chemical signals (cytokines and chemokines), and innate immune cells are the first responders to the chemicals released by the damaged tissues. This, in turn, activates the adaptive immune cells to facilitate the healing process. Macrophages are a group of innate immune cells that can kill microorganisms invading the body as well as heal wounded/damaged tissues. These are the first white blood cells to appear during embryonic development and are also among the first innate immune cells to get recruited at the site of damaged tissues. Macrophages are highly dynamic and can respond to diverse environmental factors. During tissue damage, an increase in the recruitment of immune cells is followed by pain, redness, swelling, and heat in the damaged tissues, a condition termed as ‘inflammation’. When this inflammation occurs in the absence of infection, it is considered sterile. While a lot of research has highlighted the role of macrophages in the healing of damaged tissues; how innate immune cells respond to the inflammatory chemicals released by the surrounding tissue to begin the process of inflammation and healing is still in the nascent stage of our understanding. The question is relatively difficult to answer in adult organisms since there are different immune cell types in the tissue which cross-communicate resulting in complicated responses. The embryonic immune system is, however, more simplified as they lack a mature adaptive system and comprise mainly macrophages.
The Raghavan Lab at DBT-inStem, studies embryonic skin to understand how communication between the epidermis and macrophages initiates inflammation. While the function of embryonic macrophages is not well defined and only now being elucidated, previous work from the lab in an embryonic skin inflammation model system (conditional knockout of integrin beta1 in the epidermis), showed that macrophages can respond to sterile (not triggered by infection) inflammatory cues. Integrin beta1 is an adhesion protein expressed by epidermal and dermal cells that binds these cells to the BM. The conditional ablation of integrin beta 1 in the epidermis led to disorganization of the BM that was associated with an increased number of macrophages and inflammation.
In the recent study published in Frontiers in Immunology, the authors Oindrila Bhattacharjee and Uttkarsh Ayyangar, first determined how the major cell types within the skin contributed to regulating inflammation in integrin beta1 knockout (KO) model system. Epidermis, dermal fibroblasts (major dermal cell type), and macrophages were isolated using fluorescence-activated cell sorting (FACS; a method that allows the isolation of specific cell types based on the surface expression of proteins) and performed Next Generation Sequencing (NGS) to identify specific sets of genes that are changed in the different cell types.
The NGS data suggested that upon the loss of integrin beta1, the epidermis expresses specific cytokines and chemokines (as a response to increased stress within the skin). These chemical signals, in turn, activate the macrophages such that they acquire pro-remodeling state and start expressing enzymes that break down the ECM as well as increase the expression of ECM-associated genes. Although fibroblasts have previously been shown as major hubs of ECM synthesis and deposition, surprisingly, Oindrila and Uttkarsh found that the macrophages showed a more prominent upregulation of ECM genes compared to the fibroblasts in the integrin beta 1 KO model. To further validate this, they removed macrophages using an antibody (CSF1R antibody) that prevents macrophage development and migration into the embryonic skin. In this experiment, the authors observed that upon removal of macrophages from the integrin beta1 KO embryonic skin, there was an overall reduction in the expression of ECM remodeling enzymes and, therefore, the ECM degradation was reduced. Next, the authors addressed the question ‘if epidermal-derived cytokines and chemokines drive the increased pro-remodeling activity of these macrophages’. To address this, the research team blocked the expression of a protein called cyclooxygenase-2 in the epidermis -- a key enzyme required to synthesize inflammatory cytokines and chemokines. This was done by treating mice with celecoxib which is a cyclooxygenase-2 blocker. The blocking of epidermal inflammation using celecoxib led to reduced matrix remodeling activity and decreased BM disruption by the dermal macrophages. This suggests that inflammation can drive the activation of macrophages to acquire exaggerated pro-remodeling characteristics. Taken together, this research study establishes that the crosstalk between the epidermis and the macrophage is an initial step in setting up inflammatory conditions in the skin.
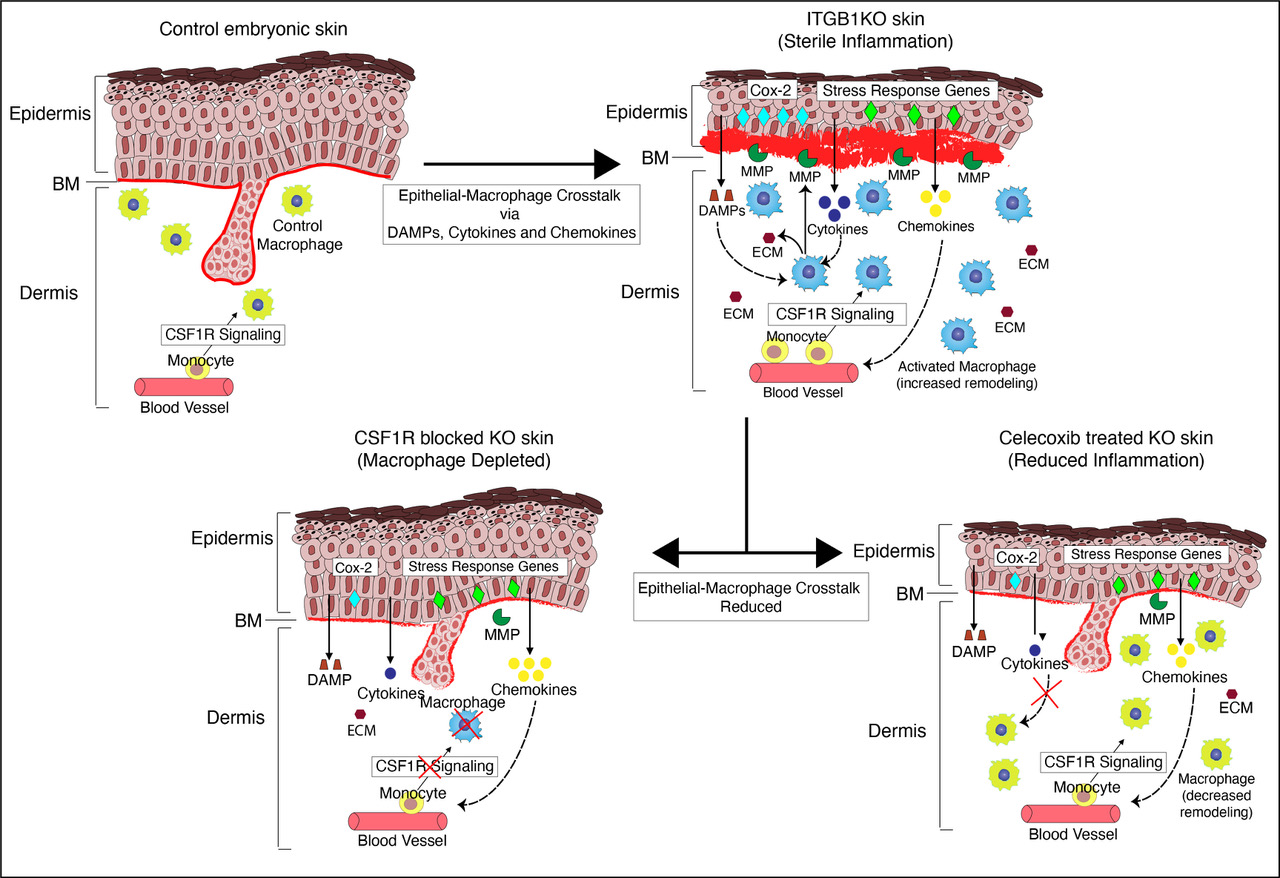
Figure: A model representing the epidermal-macrophage crosstalk in the epidermal knockout of integrin beta 1 in the embryonic skin.
MMP: Matrix Metalloproteinase (ECM remodeling enzymes); DAMPS: Damage Associated Molecular Patterns (epidermal chemical signals); ECM: Extra Cellular Matrix
Highlighting the importance of the work, Dr. Srikala Raghavan said, “In many chronic inflammatory disease conditions, the activity of adaptive immune cells is therapeutically targeted. However, this treatment strategy has profound side effects on the body’s immune system that can eventually lead to other fatal disease conditions. Hence there is a need to identify other treatment strategies to treat chronic inflammatory conditions. Our data suggest that inflammation is initiated by crosstalk between damaged cells and innate immune cells such as macrophages that cause them to acquire an enhanced pro-remodeling state. This further causes an increase in tissue damage through ECM breakdown. Our work has potential to open up a new line of therapeutics that can specifically target crosstalk between damaged cells with the innate immune cells to treat chronic inflammatory disorders.”
****
The article was written with inputs from Oindrila Bhattacharjee and Uttkarsh Ayyangar from Dr. Raghavan’s lab.
Reference:
Epithelial-Macrophage Crosstalk Initiates Sterile Inflammation in Embryonic Skin
Oindrila Bhattacharjee, Uttkarsh Ayyangar, Ambika S. Kurbet, Vairavan Lakshmanan, Dasaradhi Palakodeti, Florent Ginhoux and Srikala Raghavan. Oct 2021, Frontiers in Immunology
https://www.frontiersin.org/articles/10.3389/fimmu.2021.718005/full
Publication Date: Oct 14, 2021











