Neuron-Glia interplay in Brain Development and Disease
About The Lab
Neurological and neurodevelopmental diseases are the leading cause of disability and death worldwide. The cellular and molecular mechanisms that interplay in brain development and disease progression are largely unknown and often understudied in diseases caused by mutations (De novo or Germline) in a single gene. Moreover, the prognosis and treatment of these neurodevelopmental diseases remains elusive. Based on my research experience, our focus is on understanding how mutations in certain genes cause complex neurodevelopmental and neurological diseases which are associated with neuronal hyperexcitability, neuroinflammation, hypomyelination, impaired motor coordination and how these genes contribute to brain development and homeostasis.
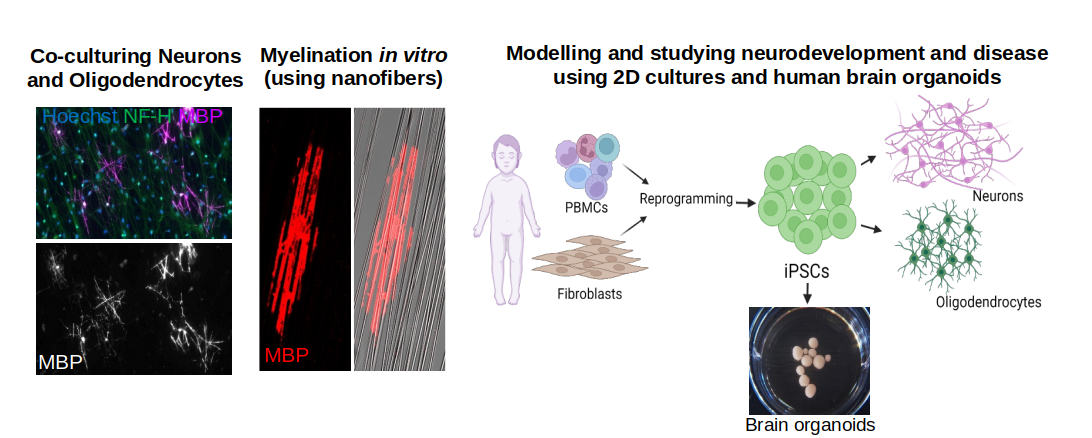
Human brain organoids (a mini brain in dish), derived from pluripotent stem cells (ESCs or iPSCs) resemble many features of the fetal human brain, including cytoarchitecture, cell diversity and maturation. Currently brain organoids gained more importance by bridging the gap between the animal models and humans to understand human brain development and etiology of many neurological disorders. In addition to conditional mice models, we use human brain organoids to comprehensively understand the risk factors and leading mechanisms contributing to disease progression. The long-term goal of my laboratory is to find possible treatment strategies by conventional drug screening or gene or stem cell therapy approaches.
Research
Our lab is focusing on understanding the complexity of brain development and molecular mechanisms that awry in neurological and neurodevelopmental disorders with particular emphasis on myelination impairment, developmental epileptic encephalopathies, and ataxia. Recessive mutations in a single gene (example PRUNE1, EPT1 and DHX37) can impact the whole brain development and disease progression in children. How these genes are implicated in brain development and disease are largely unknown. We use primary cells (NSCs, Neurons and Oligodendrocytes), conditional mouse models and human brain organoids (derived from CRISPR edited ESCs or patient derived iPSCs) as model systems to dissect the cellular (cell autonomous and non-cell autonomous) and molecular events that intricate in brain physiology, and disease progression. We use cell biology, biochemistry, molecular biology and cutting-edge techniques (Proximity ligation, Mass Spectrometry, RNA-Seq, Chip-Seq and Proteomics) to delineate the disease mechanisms and find possible therapeutic interventions by regenerative medicine approaches.



 Share
Share
 srinivas@instem.res.in
srinivas@instem.res.in 91-80-61948001 EXT, 8232
91-80-61948001 EXT, 8232
