Tubulin code, mammalian cilia and tissue homeostasis
About The Lab
Most mammalian cells contain tiny hair-like structures called cilia or flagella. These structures, once thought to be vestigial, have emerged as one of the key players in regulating diverse cellular functions. Cilia and flagella play a variety of roles in cellular signalling, chemo- and mechanosensing, organogenesis, cell and tissue homeostasis (non-motile primary cilia), as well as fluid flow and motility (motile cilia and flagella). All cilia are assembled around microtubule-based structures, the axonemes.
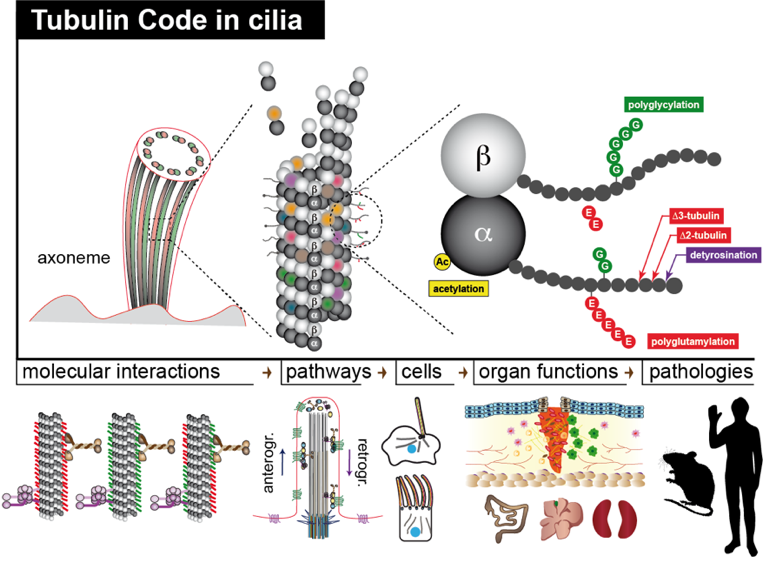
Despite their unique structure and function, axonemal microtubules are assembled from similar tubulin units as other cellular microtubules. Thus, one long-standing question is how do the microtubules adapt to their unique functions in cilia and flagella. One possible mechanism is through the posttranslational modifications (PTMs) of tubulin, which are key components of the ‘tubulin code’ which currently emerge as main regulators of MT properties and functions. The mechanism of how these tubulin PTMs regulate cilia and flagella functions is barely understood.
We are interested in understanding how specific posttranslational modifications of tubulin control diverse molecular processes within cilia and flagella, and how this in turn assures the physiological functions at cellular, organ and organism levels. The long-term goals of my team is to determine how defects in enzymes responsible for specific tubulin PTMs affect cilia function, thus leading to diverse human pathologies, called ciliopathies. Understanding these mechanisms can pave the way to future therapeutics.
We undertake a synergistic approach that involves in vitro, in cellulo and in vivo approaches combining biochemical, cell biology, molecular biology and proteomics to address the diverse questions.
Research
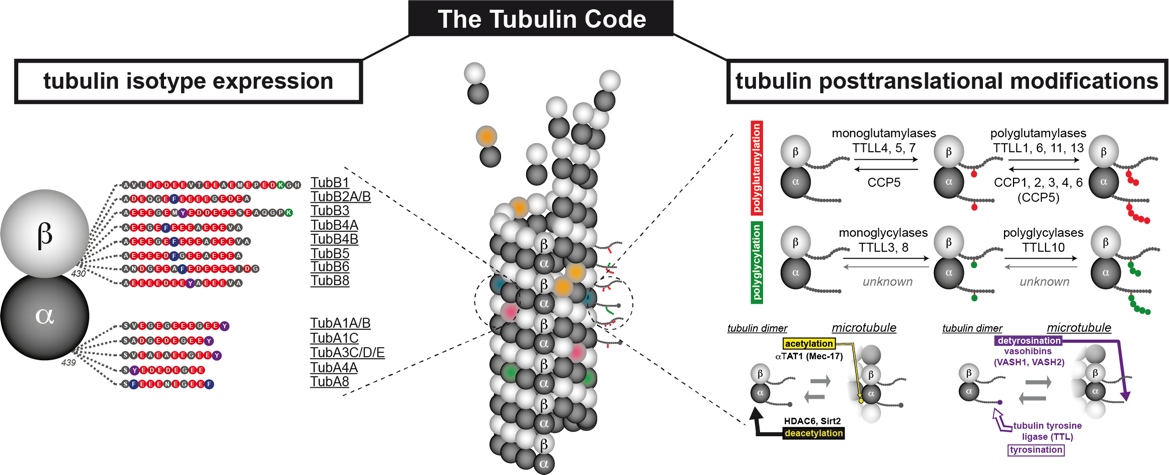
Microtubules, polymers of α- and β- tubulin dimers, undergo diverse posttranslational modifications (PTMs), which are key components of an emerging “tubulin code” that appears to play key roles in regulating microtubule properties and functions. Among the different microtubule-based structures, my interest has been the axonemes – the core structure of mammalian cilia and flagella – which are rich in tubulin PTMs and their perturbations can lead to various pathological disorders. Till date, understanding the role of tubulin PTMs in cilia has come predominantly from studies on motile cilia like the sperm flagella and the tracheal cilia with little or no understanding of the role of these PTMs in regulating functions of non‑motile primary cilia.
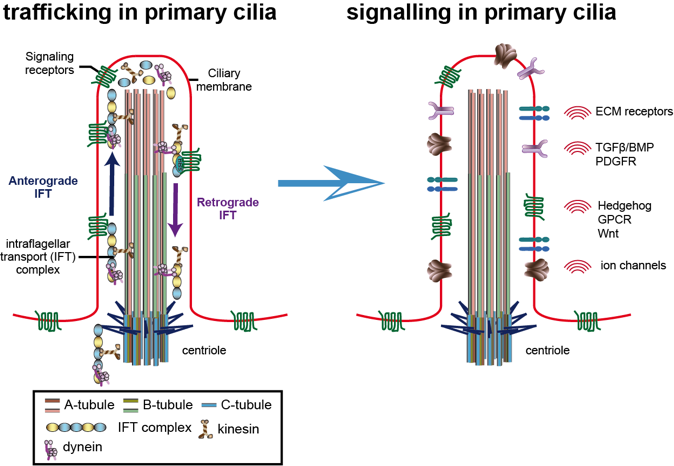
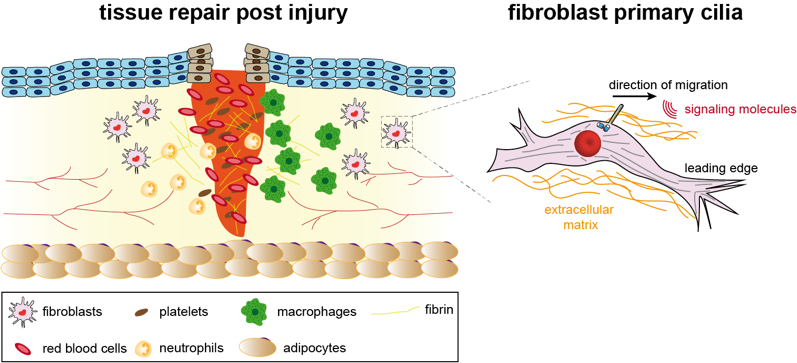
Primary cilia are a hub of signalling pathways and are key for organ development and tissue homeostasis, with defects causing multiple diseases, collectively termed ciliopathies. A major aspect of tissue homeostasis is regeneration or repair post-injury, which is a tightly regulated multi-cascade process that depends on cytoskeletal remodelling. Emerging evidence suggests that wound healing requires precise ciliogenesis and an intricate network of signalling pathways within primary cilia that control cell migration and provide directionality.
My lab is interested in understanding how the primary cilia on different cells within the tissue crosstalk to be able to regulate tissue homeostasis, especially in repair post injury/infections. A major focus of the lab is to understand how axonemal microtubules and their posttranslational modifications are modulating ciliary trafficking, thus controlling primary cilia function, which in turn would play a role in regulating tissue homeostasis.
We will be undertaking a multi-faceted approach centring around primary cilia functions and how these are affected by changes in microtubule architecture. The approach synergizes in vitro, in cellulo and in vivo analyses to address specific questions which include:
- Identifying the specific pattern of modifications on ciliary microtubules established by the different tubulin modifying enzymes, which can thus regulate specific cilia/flagella functions in mammals.
- Deciphering how two key tubulin PTMs, glutamylation and glycylation, regulate trafficking within primary cilia, thus specific signalling pathways in primary cilia.
- Determining how tubulin PTM-regulated ciliary signalling impacts organ function and tissue homeostasis.
- Establishing the mechanistic link between perturbed PTMs, cilia function and specific human disorders.
My other research interests include understanding why other cellular and ciliary proteins are also modified by the tubulin modifying enzymes. This involves an initial identification of the different proteins that can be modified by a specific enzyme, followed by understanding of the effect of the modification on the function of the protein. This will open up a new avenue for the roles of glutamylation and glycylation, beyond their role in regulating microtubule functions.



 Share
Share
 sudarshang@instem.res.in
sudarshang@instem.res.in 91-80-61948001 EXT, 8142
91-80-61948001 EXT, 8142
